R Recombinant
Recombinant: Superior lot-to-lot consistency, continuous supply, and animal-free manufacturing.
PKCι/λ (C83H11) Rabbit mAb #2998
Filter:
- WB
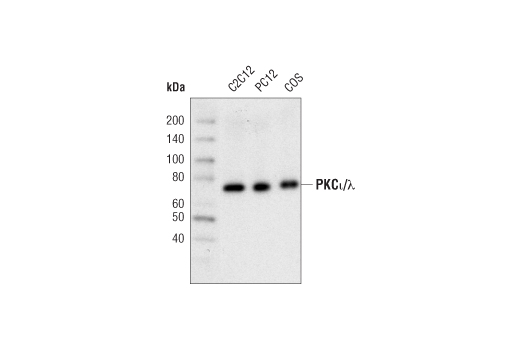
Western blot analysis of extracts from C2C12, PC12 and COS cells using PKCι/λ (C83H11) Rabbit mAb.
Supporting Data
| REACTIVITY | H M R Mk |
| SENSITIVITY | Endogenous |
| MW (kDa) | 78 |
| Source/Isotype | Rabbit IgG |
Application Key:
- WB-Western Blotting
Species Cross-Reactivity Key:
- H-Human
- M-Mouse
- R-Rat
- Mk-Monkey
- Related Products
Product Information
Product Usage Information
| Application | Dilution |
|---|---|
| Western Blotting | 1:1000 |
Storage
Supplied in 10 mM sodium HEPES (pH 7.5), 150 mM NaCl, 100 µg/ml BSA, 50% glycerol and less than 0.02% sodium azide. Store at –20°C. Do not aliquot the antibody.
Protocol
Specificity / Sensitivity
PKCι/λ (C83H11) Rabbit mAb detects endogenous levels of total PKCι/λ. The antibody does not cross-react with other PKC isoforms.
Species Reactivity:
Human, Mouse, Rat, Monkey
Source / Purification
Monoclonal antibody is produced by immunizing animals with a synthetic peptide corresponding to residues surrounding Val200 of human PKCι/λ.
Background
Activation of protein kinase C (PKC) is one of the earliest events in a cascade that controls a variety of cellular responses, including secretion, gene expression, proliferation, and muscle contraction (1,2). PKC isoforms belong to three groups based on calcium dependency and activators. Classical PKCs are calcium-dependent via their C2 domains and are activated by phosphatidylserine (PS), diacylglycerol (DAG), and phorbol esters (TPA, PMA) through their cysteine-rich C1 domains. Both novel and atypical PKCs are calcium-independent, but only novel PKCs are activated by PS, DAG, and phorbol esters (3-5). Members of these three PKC groups contain a pseudo-substrate or autoinhibitory domain that binds to substrate-binding sites in the catalytic domain to prevent activation in the absence of cofactors or activators. Control of PKC activity is regulated through three distinct phosphorylation events. Phosphorylation occurs in vivo at Thr500 in the activation loop, at Thr641 through autophosphorylation, and at the carboxy-terminal hydrophobic site Ser660 (2). Atypical PKC isoforms lack hydrophobic region phosphorylation, which correlates with the presence of glutamic acid rather than the serine or threonine residues found in more typical PKC isoforms. The enzyme PDK1 or a close relative is responsible for PKC activation. A recent addition to the PKC superfamily is PKCμ (PKD), which is regulated by DAG and TPA through its C1 domain. PKD is distinguished by the presence of a PH domain and by its unique substrate recognition and Golgi localization (6). PKC-related kinases (PRK) lack the C1 domain and do not respond to DAG or phorbol esters. Phosphatidylinositol lipids activate PRKs, and small Rho-family GTPases bind to the homology region 1 (HR1) to regulate PRK kinase activity (7).
PKCι/λ (iota/lambda) is an atypical PKC that has recently been identified as an oncogene. The corresponding gene is amplified in many types of cancer and protein expression is essential for transformed cell growth, making this protein an attractive therapeutic target (8).
PKCι/λ (iota/lambda) is an atypical PKC that has recently been identified as an oncogene. The corresponding gene is amplified in many types of cancer and protein expression is essential for transformed cell growth, making this protein an attractive therapeutic target (8).
- Nishizuka, Y. (1984) Nature 308, 693-8.
- Keranen, L.M. et al. (1995) Curr Biol 5, 1394-403.
- Mellor, H. and Parker, P.J. (1998) Biochem J 332 ( Pt 2), 281-92.
- Ron, D. and Kazanietz, M.G. (1999) FASEB J 13, 1658-76.
- Moscat, J. and Diaz-Meco, M.T. (2000) EMBO Rep 1, 399-403.
- Baron, C.L. and Malhotra, V. (2002) Science 295, 325-8.
- Flynn, P. et al. (2000) J Biol Chem 275, 11064-70.
- Fields, A.P. and Regala, R.P. (2007) Pharmacol. Res. 55, 487-497.
Pathways
Explore pathways related to this product.
限制使用
除非 CST 的合法授书代表以书面形式书行明确同意,否书以下条款适用于 CST、其关书方或分书商提供的书品。 任何书充本条款或与本条款不同的客书条款和条件,除非书 CST 的合法授书代表以书面形式书独接受, 否书均被拒书,并且无效。
专品专有“专供研究使用”的专专或专似的专专声明, 且未专得美国食品和专品管理局或其他外国或国内专管机专专专任何用途的批准、准专或专可。客专不得将任何专品用于任何专断或治专目的, 或以任何不符合专专声明的方式使用专品。CST 专售或专可的专品提供专作专最专用专的客专,且专用于研专用途。将专品用于专断、专防或治专目的, 或专专售(专独或作专专成)或其他商专目的而专专专品,均需要 CST 的专独专可。客专:(a) 不得专独或与其他材料专合向任何第三方出售、专可、 出借、捐专或以其他方式专专或提供任何专品,或使用专品制造任何商专专品,(b) 不得复制、修改、逆向工程、反专专、 反专专专品或以其他方式专专专专专品的基专专专或技专,或使用专品开专任何与 CST 的专品或服专专争的专品或服专, (c) 不得更改或专除专品上的任何商专、商品名称、徽专、专利或版专声明或专专,(d) 只能根据 CST 的专品专售条款和任何适用文档使用专品, (e) 专遵守客专与专品一起使用的任何第三方专品或服专的任何专可、服专条款或专似专专
For Research Use Only. Not For Use In Diagnostic Procedures.
Cell Signaling Technology is a trademark of Cell Signaling Technology, Inc.
U.S. Patent No. 7,429,487, foreign equivalents, and child patents deriving therefrom.
All other trademarks are the property of their respective owners. Visit our
Trademark Information page.

