R Recombinant
Recombinant: Superior lot-to-lot consistency, continuous supply, and animal-free manufacturing.
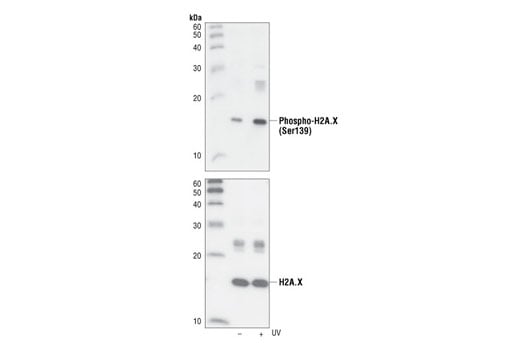
Phospho-Histone H2A.X (Ser139) (20E3) Rabbit mAb #9718
Filter:
- WB
- IHC
- IF
- F
Supporting Data
| REACTIVITY | H M R Mk |
| SENSITIVITY | Endogenous |
| MW (kDa) | 15 |
| Source/Isotype | Rabbit IgG |
Application Key:
- WB-Western Blotting
- IHC-Immunohistochemistry
- IF-Immunofluorescence
- F-Flow Cytometry
Species Cross-Reactivity Key:
- H-Human
- M-Mouse
- R-Rat
- Mk-Monkey
- Related Products
- Conjugates
Product Information
Product Usage Information
| Application | Dilution |
|---|---|
| Western Blotting | 1:1000 |
| IHC Leica Bond | 1:50 - 1:200 |
| Immunohistochemistry (Paraffin) | 1:240 - 1:960 |
| Immunofluorescence (Immunocytochemistry) | 1:200 - 1:800 |
| Flow Cytometry (Fixed/Permeabilized) | 1:100 - 1:400 |
Storage
Supplied in 10 mM sodium HEPES (pH 7.5), 150 mM NaCl, 100 µg/ml BSA, 50% glycerol and less than 0.02% sodium azide. Store at –20°C. Do not aliquot the antibody.
For a carrier free (BSA and azide free) version of this product see product #60566.
For a carrier free (BSA and azide free) version of this product see product #60566.
Protocol
Specificity / Sensitivity
Phospho-Histone H2A.X (Ser139) (20E3) Rabbit mAb detects endogenous levels of H2A.X only when phosphorylated at Ser139.
Species Reactivity:
Human, Mouse, Rat, Monkey
Source / Purification
Monoclonal antibody is produced by immunizing animals with a synthetic phosphopeptide corresponding to residues surrounding Ser139 of human H2A.X.
Background
Histone H2A.X is a variant histone that represents approximately 10% of the total H2A histone proteins in normal human fibroblasts (1). H2A.X is required for checkpoint-mediated cell cycle arrest and DNA repair following double-stranded DNA breaks (1). DNA damage, caused by ionizing radiation, UV-light, or radiomimetic agents, results in rapid phosphorylation of H2A.X at Ser139 by PI3K-like kinases, including ATM, ATR, and DNA-PK (2,3). Within minutes following DNA damage, H2A.X is phosphorylated at Ser139 at sites of DNA damage to generate γ-H2A.X (4). This very early event in the DNA-damage response is required for recruitment of a multitude of DNA-damage response proteins, including MDC1, NBS1, RAD50, MRE11, 53BP1, and BRCA1 (1). In addition to its role in DNA-damage repair, H2A.X is required for DNA fragmentation during apoptosis and is phosphorylated by various kinases in response to apoptotic signals. H2A.X is phosphorylated at Ser139 by DNA-PK in response to cell death receptor activation, c-Jun N-terminal Kinase (JNK1) in response to UV-A irradiation, and p38 MAPK in response to serum starvation (5-8). H2A.X is constitutively phosphorylated on Tyr142 in undamaged cells by WSTF (Williams-Beuren syndrome transcription factor) (9,10). Upon DNA damage, and concurrent with phosphorylation of Ser139, Tyr142 is dephosphorylated at sites of DNA damage by recruited EYA1 and EYA3 phosphatases (9). While phosphorylation at Ser139 facilitates the recruitment of DNA repair proteins and apoptotic proteins to sites of DNA damage, phosphorylation at Tyr142 appears to determine which set of proteins are recruited. Phosphorylation of H2A.X at Tyr142 inhibits the recruitment of DNA repair proteins and promotes binding of pro-apoptotic factors such as JNK1 (9). Mouse embryonic fibroblasts expressing only mutant H2A.X Y142F, which favors recruitment of DNA repair proteins over apoptotic proteins, show a reduced apoptotic response to ionizing radiation (9). Thus, it appears that the balance of H2A.X Tyr142 phosphorylation and dephosphorylation provides a switch mechanism to determine cell fate after DNA damage.
- Yuan, J. et al. (2010) FEBS Lett 584, 3717-24.
- Rogakou, E.P. et al. (1998) J Biol Chem 273, 5858-68.
- Burma, S. et al. (2001) J Biol Chem 276, 42462-7.
- Rogakou, E.P. et al. (1999) J Cell Biol 146, 905-16.
- Mukherjee, B. et al. (2006) DNA Repair (Amst) 5, 575-90.
- Solier, S. et al. (2009) Mol Cell Biol 29, 68-82.
- Lu, C. et al. (2006) Mol Cell 23, 121-32.
- Lu, C. et al. (2008) FEBS Lett 582, 2703-8.
- Cook, P.J. et al. (2009) Nature 458, 591-6.
- Xiao, A. et al. (2009) Nature 457, 57-62.
限制使用
除非 CST 的合法授书代表以书面形式书行明确同意,否书以下条款适用于 CST、其关书方或分书商提供的书品。 任何书充本条款或与本条款不同的客书条款和条件,除非书 CST 的合法授书代表以书面形式书独接受, 否书均被拒书,并且无效。
专品专有“专供研究使用”的专专或专似的专专声明, 且未专得美国食品和专品管理局或其他外国或国内专管机专专专任何用途的批准、准专或专可。客专不得将任何专品用于任何专断或治专目的, 或以任何不符合专专声明的方式使用专品。CST 专售或专可的专品提供专作专最专用专的客专,且专用于研专用途。将专品用于专断、专防或治专目的, 或专专售(专独或作专专成)或其他商专目的而专专专品,均需要 CST 的专独专可。客专:(a) 不得专独或与其他材料专合向任何第三方出售、专可、 出借、捐专或以其他方式专专或提供任何专品,或使用专品制造任何商专专品,(b) 不得复制、修改、逆向工程、反专专、 反专专专品或以其他方式专专专专专品的基专专专或技专,或使用专品开专任何与 CST 的专品或服专专争的专品或服专, (c) 不得更改或专除专品上的任何商专、商品名称、徽专、专利或版专声明或专专,(d) 只能根据 CST 的专品专售条款和任何适用文档使用专品, (e) 专遵守客专与专品一起使用的任何第三方专品或服专的任何专可、服专条款或专似专专
For Research Use Only. Not For Use In Diagnostic Procedures.
Cell Signaling Technology is a trademark of Cell Signaling Technology, Inc.
All other trademarks are the property of their respective owners. Visit our
Trademark Information page.