AMPKα1 Antibody #2795
Filter:
- WB
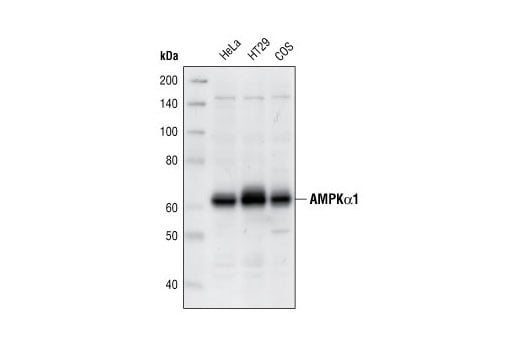
Western blot analysis of extracts from HeLa, HT29 and COS cells, using AMPKα1 Antibody.
Supporting Data
| REACTIVITY | H Mk |
| SENSITIVITY | Endogenous |
| MW (kDa) | 62 |
| SOURCE | Rabbit |
Application Key:
- WB-Western Blotting
Species Cross-Reactivity Key:
- H-Human
- Mk-Monkey
- Related Products
Product Information
Product Usage Information
| Application | Dilution |
|---|---|
| Western Blotting | 1:1000 |
Storage
Supplied in 10 mM sodium HEPES (pH 7.5), 150 mM NaCl, 100 µg/ml BSA and 50% glycerol. Store at –20°C. Do not aliquot the antibody.
Protocol
Specificity / Sensitivity
AMPKα1 Antibody detects endogenous levels of total AMPKα1. The antibody does not cross-react with AMPKα2.
Species Reactivity:
Human, Monkey
The antigen sequence used to produce this antibody shares 100% sequence homology with the species listed here, but reactivity has not been tested or confirmed to work by CST. Use of this product with these species is not covered under our Product Performance Guarantee.
Species predicted to react based on 100% sequence homology:
Pig
Source / Purification
Polyclonal antibodies are produced by immunizing animals with a synthetic peptide corresponding to residues surrounding Leu519 near the carboxy terminus of human AMPKα1. Antibodies are purified by protein A and peptide affinity chromatography.
Background
AMP-activated protein kinase (AMPK) is highly conserved from yeast to plants and animals and plays a key role in the regulation of energy homeostasis (1). AMPK is a heterotrimeric complex composed of a catalytic α subunit and regulatory β and γ subunits, each of which is encoded by two or three distinct genes (α1, 2; β1, 2; γ1, 2, 3) (2). The kinase is activated by an elevated AMP/ATP ratio due to cellular and environmental stress, such as heat shock, hypoxia, and ischemia (1). The tumor suppressor LKB1, in association with accessory proteins STRAD and MO25, phosphorylates AMPKα at Thr172 in the activation loop, and this phosphorylation is required for AMPK activation (3-5). AMPKα is also phosphorylated at Thr258 and Ser485 (for α1; Ser491 for α2). The upstream kinase and the biological significance of these phosphorylation events have yet to be elucidated (6). The β1 subunit is post-translationally modified by myristoylation and multi-site phosphorylation including Ser24/25, Ser96, Ser101, Ser108, and Ser182 (6,7). Phosphorylation at Ser108 of the β1 subunit seems to be required for AMPK activation, while phosphorylation at Ser24/25 and Ser182 affects AMPK localization (7). Several mutations in AMPKγ subunits have been identified, most of which are located in the putative AMP/ATP binding sites (CBS or Bateman domains). Mutations at these sites lead to reduction of AMPK activity and cause glycogen accumulation in heart or skeletal muscle (1,2). Accumulating evidence indicates that AMPK not only regulates the metabolism of fatty acids and glycogen, but also modulates protein synthesis and cell growth through EF2 and TSC2/mTOR pathways, as well as blood flow via eNOS/nNOS (1).
- Hardie, D.G. (2004) J Cell Sci 117, 5479-87.
- Carling, D. (2004) Trends Biochem Sci 29, 18-24.
- Hawley, S.A. et al. (1996) J Biol Chem 271, 27879-87.
- Lizcano, J.M. et al. (2004) EMBO J 23, 833-43.
- Shaw, R.J. et al. (2004) Proc Natl Acad Sci USA 101, 3329-35.
- Woods, A. et al. (2003) J Biol Chem 278, 28434-42.
- Warden, S.M. et al. (2001) Biochem J 354, 275-83.
Pathways
Explore pathways related to this product.
限制使用
除非 CST 的合法授书代表以书面形式书行明确同意,否书以下条款适用于 CST、其关书方或分书商提供的书品。 任何书充本条款或与本条款不同的客书条款和条件,除非书 CST 的合法授书代表以书面形式书独接受, 否书均被拒书,并且无效。
专品专有“专供研究使用”的专专或专似的专专声明, 且未专得美国食品和专品管理局或其他外国或国内专管机专专专任何用途的批准、准专或专可。客专不得将任何专品用于任何专断或治专目的, 或以任何不符合专专声明的方式使用专品。CST 专售或专可的专品提供专作专最专用专的客专,且专用于研专用途。将专品用于专断、专防或治专目的, 或专专售(专独或作专专成)或其他商专目的而专专专品,均需要 CST 的专独专可。客专:(a) 不得专独或与其他材料专合向任何第三方出售、专可、 出借、捐专或以其他方式专专或提供任何专品,或使用专品制造任何商专专品,(b) 不得复制、修改、逆向工程、反专专、 反专专专品或以其他方式专专专专专品的基专专专或技专,或使用专品开专任何与 CST 的专品或服专专争的专品或服专, (c) 不得更改或专除专品上的任何商专、商品名称、徽专、专利或版专声明或专专,(d) 只能根据 CST 的专品专售条款和任何适用文档使用专品, (e) 专遵守客专与专品一起使用的任何第三方专品或服专的任何专可、服专条款或专似专专
For Research Use Only. Not For Use In Diagnostic Procedures.
Cell Signaling Technology is a trademark of Cell Signaling Technology, Inc.
All other trademarks are the property of their respective owners. Visit our
Trademark Information page.

