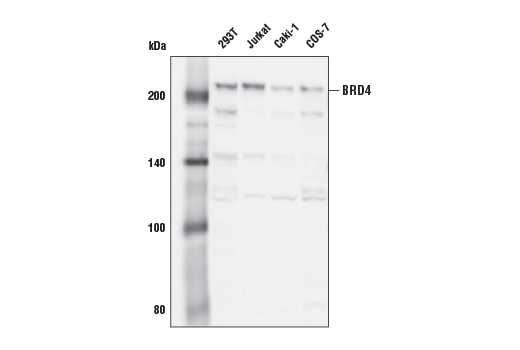
BRD4 (E4X7E) Mouse mAb #63759
Filter:
- WB
- IP
- IF
Supporting Data
| REACTIVITY | H Mk |
| SENSITIVITY | Endogenous |
| MW (kDa) | 200 |
| Source/Isotype | Mouse IgG1 |
Application Key:
- WB-Western Blotting
- IP-Immunoprecipitation
- IF-Immunofluorescence
Species Cross-Reactivity Key:
- H-Human
- Mk-Monkey
- Related Products
Product Information
Product Usage Information
| Application | Dilution |
|---|---|
| Western Blotting | 1:1000 |
| Simple Western™ | 1:50 - 1:250 |
| Immunoprecipitation | 1:100 |
| Immunofluorescence (Immunocytochemistry) | 1:1600 - 1:6400 |
Storage
Supplied in 10 mM sodium HEPES (pH 7.5), 150 mM NaCl, 100 µg/ml BSA, 50% glycerol and less than 0.02% sodium azide. Store at –20°C. Do not aliquot the antibody.
Protocol
Specificity / Sensitivity
BRD4 (E4X7E) Mouse mAb recognizes endogenous levels of total BRD4 protein. This antibody specifically recognizes the BRD4 long isoform (UniProt #O60885-1) and does not recognize other BRD4 isoforms.
Species Reactivity:
Human, Monkey
Source / Purification
Monoclonal antibody is produced by immunizing animals with a synthetic peptide corresponding to residues surrounding Leu1129 of human BRD4 protein.
Background
Bromodomain-containing protein 4 (BRD4) is a member of the bromodomains and extra terminal (BET) family of proteins, which also includes BRD2, BRD3, and BRDT (1-3). BET family proteins contain two tandem bromodomains and an extra terminal (ET) domain, and bind acetyl lysine residues (3). BRD4 is a chromatin-binding protein with a preference for Lys14 on histone H3 as well as Lys5 and Lys12 on histone H4 (4). BRD4 chromatin binding occurs throughout the cell cycle, including condensed mitotic chromosomes, when the majority of genes are silenced (5). BRD4 association with chromatin during mitosis is thought to be an important part of the bookmarking mechanism to accelerate reactivation of the silenced genes upon exit from mitosis (2,6). BRD4 has been shown to facilitate transcription by recruiting the positive transcription elongation factor b (pTEFb) complex that phosphorylates Ser2 of the heptapeptide repeat of the carboxy-terminal domain of RNA polymerase II, promoting transcription elongation (3,7,8). In addition, BRD4 has been found to be part of the super elongation complex and the polymerase associated factor complex (PAFc) in MLL-fusion derived leukemia cell lines, demonstrating a role for BRD4 in the regulation of transcription elongation (9). Research studies have shown that BRD4 (and BET family proteins) may be promising therapeutic targets for various Myc-driven cancers, such as Burkitt’s lymphoma and certain acute myeloid leukemias (1,10,11). Investigators have found molecular inhibition of BET proteins to be effective in inducing apoptosis in various MLL-fusion driven leukemic cell lines by competing BRD3 and BRD4 from chromatin, leading to reduced expression of Bcl-2, Myc, and CDK6 (9). BET inhibition has also been shown to have antitumor activities against nuclear protein in testis (NUT) midline carcinoma cell lines and xenografts in mice where BRD4 is found to be a frequent translocation partner of the NUT protein (12). In addition, BRD4 regulates the expression of some inflammatory genes, and inhibition of BRD4 (and BET family proteins) chromatin binding causes reduced expression of a subset of inflammatory genes in macrophages, leading to protection against endotoxic shock and sepsis (13).
- Belkina, A.C. and Denis, G.V. (2012) Nat Rev Cancer 12, 465-77.
- Voigt, P. and Reinberg, D. (2011) Genome Biol 12, 133.
- Wu, S.Y. and Chiang, C.M. (2007) J Biol Chem 282, 13141-5.
- Dey, A. et al. (2003) Proc Natl Acad Sci U S A 100, 8758-63.
- Dey, A. et al. (2009) Mol Biol Cell 20, 4899-909.
- Zhao, R. et al. (2011) Nat Cell Biol 13, 1295-304.
- Jang, M.K. et al. (2005) Mol Cell 19, 523-34.
- Yang, Z. et al. (2005) Mol Cell 19, 535-45.
- Dawson, M.A. et al. (2011) Nature 478, 529-33.
- Muller, S. et al. (2011) Expert Rev Mol Med 13, e29.
- Mertz, J.A. et al. (2011) Proc Natl Acad Sci U S A 108, 16669-74.
- Filippakopoulos, P. et al. (2010) Nature 468, 1067-73.
- Nicodeme, E. et al. (2010) Nature 468, 1119-23.
限制使用
除非 CST 的合法授书代表以书面形式书行明确同意,否书以下条款适用于 CST、其关书方或分书商提供的书品。 任何书充本条款或与本条款不同的客书条款和条件,除非书 CST 的合法授书代表以书面形式书独接受, 否书均被拒书,并且无效。
专品专有“专供研究使用”的专专或专似的专专声明, 且未专得美国食品和专品管理局或其他外国或国内专管机专专专任何用途的批准、准专或专可。客专不得将任何专品用于任何专断或治专目的, 或以任何不符合专专声明的方式使用专品。CST 专售或专可的专品提供专作专最专用专的客专,且专用于研专用途。将专品用于专断、专防或治专目的, 或专专售(专独或作专专成)或其他商专目的而专专专品,均需要 CST 的专独专可。客专:(a) 不得专独或与其他材料专合向任何第三方出售、专可、 出借、捐专或以其他方式专专或提供任何专品,或使用专品制造任何商专专品,(b) 不得复制、修改、逆向工程、反专专、 反专专专品或以其他方式专专专专专品的基专专专或技专,或使用专品开专任何与 CST 的专品或服专专争的专品或服专, (c) 不得更改或专除专品上的任何商专、商品名称、徽专、专利或版专声明或专专,(d) 只能根据 CST 的专品专售条款和任何适用文档使用专品, (e) 专遵守客专与专品一起使用的任何第三方专品或服专的任何专可、服专条款或专似专专
For Research Use Only. Not For Use In Diagnostic Procedures.
Cell Signaling Technology is a trademark of Cell Signaling Technology, Inc.
All other trademarks are the property of their respective owners. Visit our
Trademark Information page.