EGF Receptor Control Cell Extracts #5634
Filter:
- WB
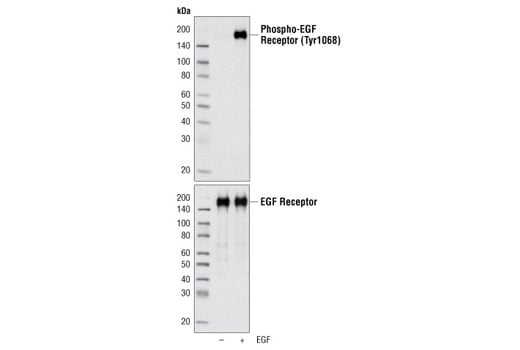
Western blot analysis of EGF Receptor Control Cell extracts using Phospho-EGF Receptor (Tyr1068) (D7A5) XP® Rabbit mAb #3777 (upper) and EGF Receptor (D38B1) XP® Rabbit mAb #4267 (lower).
- Product Includes
- Related Products
| Product Includes | Quantity |
|---|---|
| EGF Receptor Control Cell Extracts (A431 untreated) #91047 | 150 µl |
| EGF Receptor Control Cell Extracts (A431 +EGF) #20417 | 150 µl |
Product Information
Product Usage Information
Boil for 3 minutes prior to use. Load 15 µl of phosphorylated and nonphosphorylated EGF Receptor Control Cell Extracts per lane.
Storage
Supplied in SDS Sample Buffer: 62.5 mM Tris-HCl (pH 6.8 at 25°C), 2% w/v SDS, 10% glycerol, 50 mM DTT, 0.01% w/v phenol red or bromophenol blue. Store at –20°C or at –80°C for long term storage.
Product Description
Nonphosphorylated EGF Receptor Control Cell Extracts: Total extracts from A431 cells, serum starved overnight to serve as a negative control. Supplied in SDS Sample Buffer.
Phosphorylated EGF Receptor Control Cell Extracts: Total extracts from A431 cells, serum starved overnight and treated with 100 ng/ml hEGF #8916 for five minutes to serve as a positive control. Supplied in SDS Sample Buffer.
Phosphorylated EGF Receptor Control Cell Extracts: Total extracts from A431 cells, serum starved overnight and treated with 100 ng/ml hEGF #8916 for five minutes to serve as a positive control. Supplied in SDS Sample Buffer.
Background
The epidermal growth factor (EGF) receptor is a transmembrane tyrosine kinase that belongs to the HER/ErbB protein family. Ligand binding results in receptor dimerization, autophosphorylation, activation of downstream signaling, internalization, and lysosomal degradation (1,2). Phosphorylation of EGF receptor (EGFR) at Tyr845 in the kinase domain is implicated in stabilizing the activation loop, maintaining the active state enzyme, and providing a binding surface for substrate proteins (3,4). c-Src is involved in phosphorylation of EGFR at Tyr845 (5). The SH2 domain of PLCγ binds at phospho-Tyr992, resulting in activation of PLCγ-mediated downstream signaling (6). Phosphorylation of EGFR at Tyr1045 creates a major docking site for the adaptor protein c-Cbl, leading to receptor ubiquitination and degradation following EGFR activation (7,8). The GRB2 adaptor protein binds activated EGFR at phospho-Tyr1068 (9). A pair of phosphorylated EGFR residues (Tyr1148 and Tyr1173) provide a docking site for the Shc scaffold protein, with both sites involved in MAP kinase signaling activation (2). Phosphorylation of EGFR at specific serine and threonine residues attenuates EGFR kinase activity. EGFR carboxy-terminal residues Ser1046 and Ser1047 are phosphorylated by CaM kinase II; mutation of either of these serines results in upregulated EGFR tyrosine autophosphorylation (10).
- Hackel, P.O. et al. (1999) Curr Opin Cell Biol 11, 184-9.
- Zwick, E. et al. (1999) Trends Pharmacol Sci 20, 408-12.
- Cooper, J.A. and Howell, B. (1993) Cell 73, 1051-4.
- Hubbard, S.R. et al. (1994) Nature 372, 746-54.
- Biscardi, J.S. et al. (1999) J Biol Chem 274, 8335-43.
- Emlet, D.R. et al. (1997) J Biol Chem 272, 4079-86.
- Levkowitz, G. et al. (1999) Mol Cell 4, 1029-40.
- Ettenberg, S.A. et al. (1999) Oncogene 18, 1855-66.
- Rojas, M. et al. (1996) J Biol Chem 271, 27456-61.
- Feinmesser, R.L. et al. (1999) J Biol Chem 274, 16168-73.
Pathways
Explore pathways related to this product.
限制使用
除非 CST 的合法授书代表以书面形式书行明确同意,否书以下条款适用于 CST、其关书方或分书商提供的书品。 任何书充本条款或与本条款不同的客书条款和条件,除非书 CST 的合法授书代表以书面形式书独接受, 否书均被拒书,并且无效。
专品专有“专供研究使用”的专专或专似的专专声明, 且未专得美国食品和专品管理局或其他外国或国内专管机专专专任何用途的批准、准专或专可。客专不得将任何专品用于任何专断或治专目的, 或以任何不符合专专声明的方式使用专品。CST 专售或专可的专品提供专作专最专用专的客专,且专用于研专用途。将专品用于专断、专防或治专目的, 或专专售(专独或作专专成)或其他商专目的而专专专品,均需要 CST 的专独专可。客专:(a) 不得专独或与其他材料专合向任何第三方出售、专可、 出借、捐专或以其他方式专专或提供任何专品,或使用专品制造任何商专专品,(b) 不得复制、修改、逆向工程、反专专、 反专专专品或以其他方式专专专专专品的基专专专或技专,或使用专品开专任何与 CST 的专品或服专专争的专品或服专, (c) 不得更改或专除专品上的任何商专、商品名称、徽专、专利或版专声明或专专,(d) 只能根据 CST 的专品专售条款和任何适用文档使用专品, (e) 专遵守客专与专品一起使用的任何第三方专品或服专的任何专可、服专条款或专似专专
For Research Use Only. Not For Use In Diagnostic Procedures.
Cell Signaling Technology is a trademark of Cell Signaling Technology, Inc.
All other trademarks are the property of their respective owners. Visit our
Trademark Information page.

