R Recombinant
Recombinant: Superior lot-to-lot consistency, continuous supply, and animal-free manufacturing.
Phospho-Stat1 (Tyr701) (58D6) Rabbit mAb (Alexa Fluor® 700 Conjugate) #76285
Filter:
- F
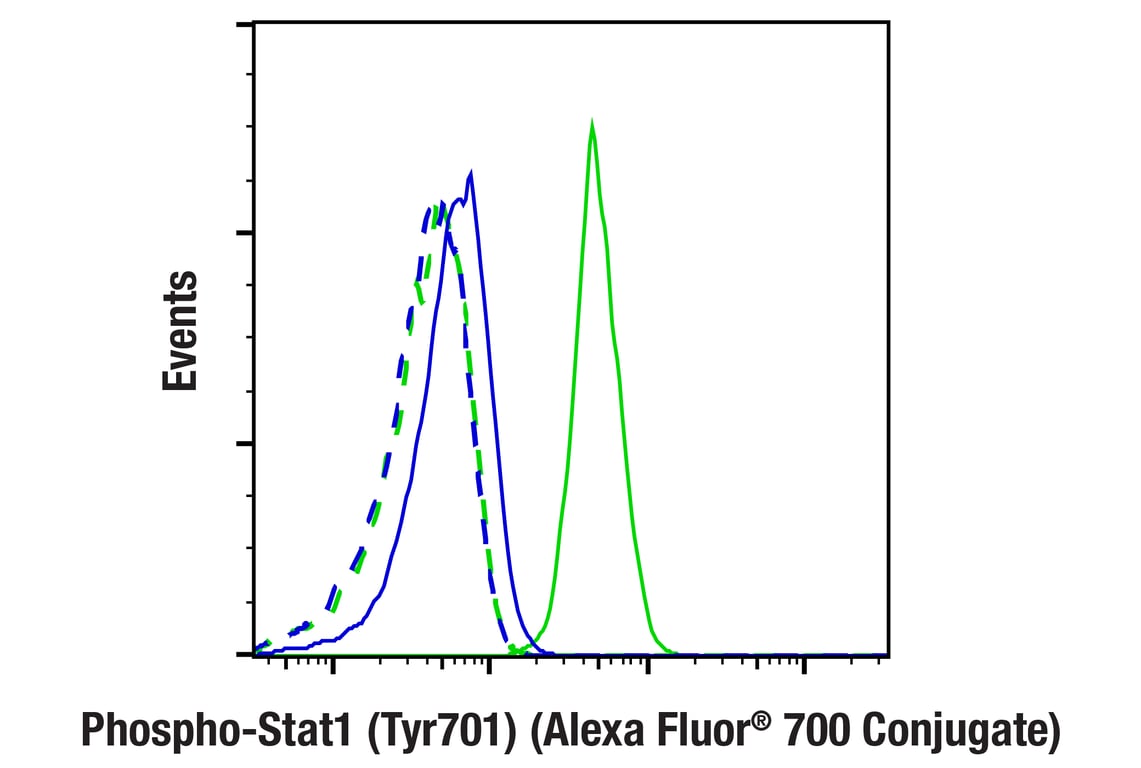
Flow cytometric analysis of Jurkat cells, untreated (blue) or treated with Human Interferon-α1 (hIFN-α1) (100 ng/mL, 5 min; green), using Phospho-Stat1 (Tyr701) (58D6) Rabbit mAb (Alexa Fluor® 700 Conjugate) (solid lines) or concentration-matched Rabbit (DA1E) mAb IgG XP® Isotype Control (Alexa Fluor® 700 Conjugate) #30720 (dashed lines).
Supporting Data
| REACTIVITY | H M |
| SENSITIVITY | Endogenous |
| MW (kDa) | |
| Source/Isotype | Rabbit IgG |
Application Key:
- F-Flow Cytometry
Species Cross-Reactivity Key:
- H-Human
- M-Mouse
- Related Products
Product Information
Product Description
This Cell Signaling Technology antibody is conjugated to Alexa Fluor® 700 fluorescent dye under optimal conditions and tested in-house for direct flow cytometric analysis in human cells. This antibody conjugate is expected to exhibit the same species cross-reactivity as the unconjugated Phospho-Stat1 (Tyr701) (58D6) Rabbit mAb #9167.
Product Usage Information
| Application | Dilution |
|---|---|
| Flow Cytometry (Fixed/Permeabilized) | 1:50 |
Storage
Supplied in PBS (pH 7.2), less than 0.1% sodium azide, and 2 mg/mL BSA. Store at 4°C. Do not aliquot the antibody. Protect from light. Do not freeze.
Protocol
Specificity / Sensitivity
Phospho-Stat1 (Tyr701) (58D6) Rabbit mAb (Alexa Fluor® 700 Conjugate) detects endogenous levels of Stat1 only when phosphorylated at Tyr701. The antibody detects phosphorylated Tyr701 of p91 Stat1 and also the p84 splice variant. It does not cross-react with the corresponding phospho-tyrosines of other Stat proteins.
Species Reactivity:
Human, Mouse
Source / Purification
Monoclonal antibody is produced by immunizing animals with a synthetic phosphopeptide corresponding to residues surrounding Tyr701 of human Stat1.
Background
The Stat1 transcription factor is activated in response to a large number of ligands (1) and is essential for responsiveness to IFN-α and IFN-γ (2,3). Phosphorylation of Stat1 at Tyr701 induces Stat1 dimerization, nuclear translocation, and DNA binding (4). Stat1 protein exists as a pair of isoforms, Stat1α (91 kDa) and the splice variant Stat1β (84 kDa). In most cells, both isoforms are activated by IFN-α, but only Stat1α is activated by IFN-γ. The inappropriate activation of Stat1 occurs in many tumors (5). In addition to tyrosine phosphorylation, Stat1 is also phosphorylated at Ser727 through a p38 mitogen-activated protein kinase (MAPK)-dependent pathway in response to IFN-α and other cellular stresses (6). Serine phosphorylation may be required for the maximal induction of Stat1-mediated gene activation.
Pathways
Explore pathways related to this product.
限制使用
除非 CST 的合法授书代表以书面形式书行明确同意,否书以下条款适用于 CST、其关书方或分书商提供的书品。 任何书充本条款或与本条款不同的客书条款和条件,除非书 CST 的合法授书代表以书面形式书独接受, 否书均被拒书,并且无效。
专品专有“专供研究使用”的专专或专似的专专声明, 且未专得美国食品和专品管理局或其他外国或国内专管机专专专任何用途的批准、准专或专可。客专不得将任何专品用于任何专断或治专目的, 或以任何不符合专专声明的方式使用专品。CST 专售或专可的专品提供专作专最专用专的客专,且专用于研专用途。将专品用于专断、专防或治专目的, 或专专售(专独或作专专成)或其他商专目的而专专专品,均需要 CST 的专独专可。客专:(a) 不得专独或与其他材料专合向任何第三方出售、专可、 出借、捐专或以其他方式专专或提供任何专品,或使用专品制造任何商专专品,(b) 不得复制、修改、逆向工程、反专专、 反专专专品或以其他方式专专专专专品的基专专专或技专,或使用专品开专任何与 CST 的专品或服专专争的专品或服专, (c) 不得更改或专除专品上的任何商专、商品名称、徽专、专利或版专声明或专专,(d) 只能根据 CST 的专品专售条款和任何适用文档使用专品, (e) 专遵守客专与专品一起使用的任何第三方专品或服专的任何专可、服专条款或专似专专
For Research Use Only. Not For Use In Diagnostic Procedures.
Cell Signaling Technology is a trademark of Cell Signaling Technology, Inc.
Alexa Fluor is a registered trademark of Life Technologies Corporation.
This product is provided under an intellectual property license from Life Technologies Corporation. The transfer of this product is conditioned on the buyer using the purchased product solely in research conducted by the buyer, excluding contract research or any fee for service research, and the buyer must not (1) use this product or its components for (a) diagnostic, therapeutic or prophylactic purposes; (b) testing, analysis or screening services, or information in return for compensation on a per-test basis; or (c) manufacturing or quality assurance or quality control, and/or (2) sell or transfer this product or its components for resale, whether or not resold for use in research. For information on purchasing a license to this product for purposes other than as described above, contact Life Technologies Corporation, 5791 Van Allen Way, Carlsbad, CA 92008 USA or outlicensing@lifetech.com.
All other trademarks are the property of their respective owners. Visit our
Trademark Information page.

