R Recombinant
Recombinant: Superior lot-to-lot consistency, continuous supply, and animal-free manufacturing.
Di-Methyl-Histone H3 (Lys36) (C75H12) Rabbit mAb #2901
Filter:
- WB
- IHC
- IF
- F
Supporting Data
| REACTIVITY | H M R Mk |
| SENSITIVITY | Endogenous |
| MW (kDa) | 17 |
| Source/Isotype | Rabbit IgG |
Application Key:
- WB-Western Blotting
- IHC-Immunohistochemistry
- IF-Immunofluorescence
- F-Flow Cytometry
Species Cross-Reactivity Key:
- H-Human
- M-Mouse
- R-Rat
- Mk-Monkey
- Related Products
- Conjugates
Product Information
Product Usage Information
| Application | Dilution |
|---|---|
| Western Blotting | 1:1000 |
| Immunohistochemistry (Paraffin) | 1:50 |
| Immunofluorescence (Immunocytochemistry) | 1:800 - 1:1600 |
| Flow Cytometry (Fixed/Permeabilized) | 1:50 |
Storage
Supplied in 10 mM sodium HEPES (pH 7.5), 150 mM NaCl, 100 µg/ml BSA, 50% glycerol and less than 0.02% sodium azide. Store at –20°C. Do not aliquot the antibody.
For a carrier free (BSA and azide free) version of this product see product #26917.
For a carrier free (BSA and azide free) version of this product see product #26917.
Protocol
Specificity / Sensitivity
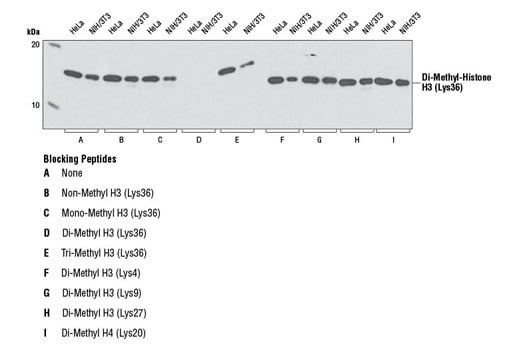
Di-Methyl-Histone H3 (Lys36) (C75H12) Rabbit mAb detects endogenous levels of histone H3.1, histone H3.2, and histone H3.3, only when di-methylated on Lys36. The antibody does not cross-react with non-methylated, mono-methylated, or tri-methylated Lys36. In addition, the antibody does not cross-react with di-methylated histone H3 Lys4, Lys9, Lys27, Lys79 or di-methylated histone H4 Lys20.
Species Reactivity:
Human, Mouse, Rat, Monkey
Source / Purification
Monoclonal antibody is produced by immunizing animals with a synthetic peptide corresponding to the amino terminus of histone H3 in which Lys36 is di-methylated.
Background
The nucleosome, made up of four core histone proteins (H2A, H2B, H3, and H4), is the primary building block of chromatin. Originally thought to function as a static scaffold for DNA packaging, histones have now been shown to be dynamic proteins, undergoing multiple types of post-translational modifications, including acetylation, phosphorylation, methylation, and ubiquitination (1). Histone methylation is a major determinant for the formation of active and inactive regions of the genome and is crucial for the proper programming of the genome during development (2,3). Arginine methylation of histones H3 (Arg2, 17, 26) and H4 (Arg3) promotes transcriptional activation and is mediated by a family of protein arginine methyltransferases (PRMTs), including the co-activators PRMT1 and CARM1 (PRMT4) (4). In contrast, a more diverse set of histone lysine methyltransferases has been identified, all but one of which contain a conserved catalytic SET domain originally identified in the Drosophila Su(var)3-9, Enhancer of zeste, and Trithorax proteins. Lysine methylation occurs primarily on histones H3 (Lys4, 9, 27, 36, 79) and H4 (Lys20) and has been implicated in both transcriptional activation and silencing (4). Methylation of these lysine residues coordinates the recruitment of chromatin modifying enzymes containing methyl-lysine binding modules such as chromodomains (HP1, PRC1), PHD fingers (BPTF, ING2), tudor domains (53BP1), and WD-40 domains (WDR5) (5-8). The discovery of histone demethylases, such as PADI4, LSD1, JMJD1, JMJD2, and JHDM1, has shown that methylation is a reversible epigenetic marker (9).
- Peterson, C.L. and Laniel, M.A. (2004) Curr Biol 14, R546-51.
- Kubicek, S. et al. (2006) Ernst Schering Res Found Workshop, 1-27.
- Lin, W. and Dent, S.Y. (2006) Curr Opin Genet Dev 16, 137-42.
- Lee, D.Y. et al. (2005) Endocr Rev 26, 147-70.
- Daniel, J.A. et al. (2005) Cell Cycle 4, 919-26.
- Shi, X. et al. (2006) Nature 442, 96-9.
- Wysocka, J. et al. (2006) Nature 442, 86-90.
- Wysocka, J. et al. (2005) Cell 121, 859-72.
- Trojer, P. and Reinberg, D. (2006) Cell 125, 213-7.
Pathways
Explore pathways related to this product.
限制使用
除非 CST 的合法授书代表以书面形式书行明确同意,否书以下条款适用于 CST、其关书方或分书商提供的书品。 任何书充本条款或与本条款不同的客书条款和条件,除非书 CST 的合法授书代表以书面形式书独接受, 否书均被拒书,并且无效。
专品专有“专供研究使用”的专专或专似的专专声明, 且未专得美国食品和专品管理局或其他外国或国内专管机专专专任何用途的批准、准专或专可。客专不得将任何专品用于任何专断或治专目的, 或以任何不符合专专声明的方式使用专品。CST 专售或专可的专品提供专作专最专用专的客专,且专用于研专用途。将专品用于专断、专防或治专目的, 或专专售(专独或作专专成)或其他商专目的而专专专品,均需要 CST 的专独专可。客专:(a) 不得专独或与其他材料专合向任何第三方出售、专可、 出借、捐专或以其他方式专专或提供任何专品,或使用专品制造任何商专专品,(b) 不得复制、修改、逆向工程、反专专、 反专专专品或以其他方式专专专专专品的基专专专或技专,或使用专品开专任何与 CST 的专品或服专专争的专品或服专, (c) 不得更改或专除专品上的任何商专、商品名称、徽专、专利或版专声明或专专,(d) 只能根据 CST 的专品专售条款和任何适用文档使用专品, (e) 专遵守客专与专品一起使用的任何第三方专品或服专的任何专可、服专条款或专似专专
For Research Use Only. Not For Use In Diagnostic Procedures.
Cell Signaling Technology is a trademark of Cell Signaling Technology, Inc.
U.S. Patent No. 7,429,487, foreign equivalents, and child patents deriving therefrom.
All other trademarks are the property of their respective owners. Visit our
Trademark Information page.